Rapidly screening for Ebola at U.S. hospitals has just gotten easier thanks to military-funded technology. Salt Lake City-based BioFire announced over the weekend that they had received emergency use authorization from the Food and Drug Administration for hospital workers to use their polymerase chain reaction (PCR) screening machine, the FilmArray, to screen for Ebola.
It’s the same machine that the U.S. military is using to fight the disease in Africa. But until Saturday, the FDA approved FilmArray for Ebola screening for research purposes only in U.S. hospitals.
The Dallas hospital that treated Thomas Eric Duncan, the Liberian man who became the first individual to die of Ebola in the United States, but was not authorized to use it to test patients showing symptoms of Ebola.
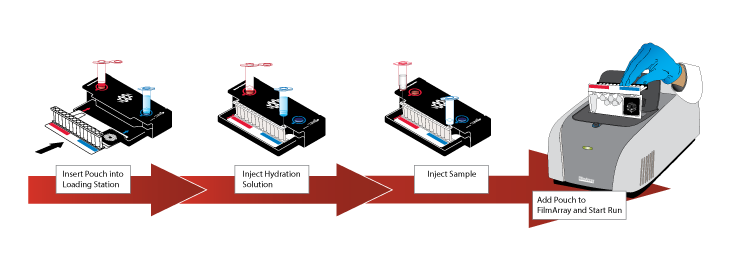
The FilmArray: User-Friendly Multiplex PCR delivers results in one hour.
Health workers in more than 300 hospitals around the country, including 30 lab hospitals in the New York tri-state area, use the machine to screen for a wide variety of illnesses such as listeria, influenza and various other respiratory and intestinal sicknesses. With the right diagnostic kit in place, the FilmArray can also be used to screen for Ebola, delivering results in about an hour with higher than 90 percent certainty. More importantly, it can do so days before an Ebola carrier develops a fever and becomes contagious. One of the hospitals scheduled to receive the machine for that purpose is New York's Bellevue.
Doctors and medical staff at Emory University in Atlanta were able to use it to diagnose and treat U.S. doctor Kent Brantly and aid worker Nancy Writebol because the machine there had a research designation.
“We understand the importance of quickly diagnosing Ebola cases in the U.S. and abroad. FDA is committed to working with companies in the most expedited manner to increase the availability of authorized diagnostic tests for Ebola for emergency use during this epidemic,” an FDA spokesperson told Defense One.
Read More at Defense One: